Fighting Infections with HMOs: Results from a Randomized Control Trial
Highlights
This clinical summary explains that human milk oligosaccharides (HMOs) reduce the occurrence of infant morbidities such as bronchitis and lower respiratory tract infections (LRTIs) and also reduces medication usage such as antibiotics and antipyretics.1
Background
HMOs have varied structures, types, and concentrations in human milk.1 2′-fucosyllactose (2’-FL) is one of the most abundant HMO, with a concentration of 10-15 g/L in mature milk.1,2 HMOs are crucial in promoting and maintaining the growth of gut flora and strengthening the immune system.1 2’-FL is also known to support the growth of certain microbiome species.1
Objective
To assess the role of HMO such as 2’-FL in reducing the incidence of bronchitis, LRTIs, and lesser use of antibiotics and antipyretic medications.
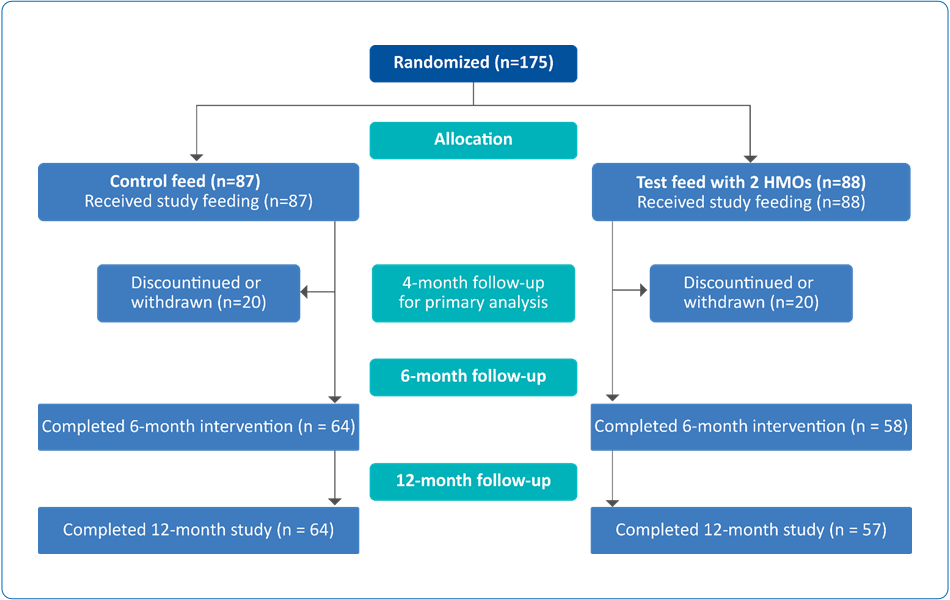
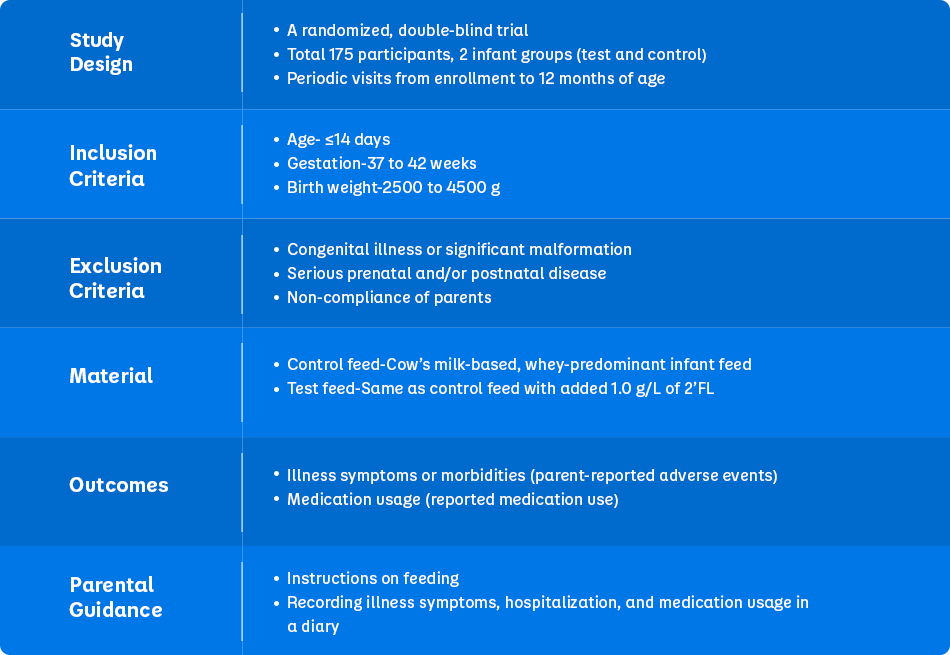
Study Design
HMOs: Human milk oligosaccharides
Table 1. Study description (Adapted from Puccio G et al., 2017)1
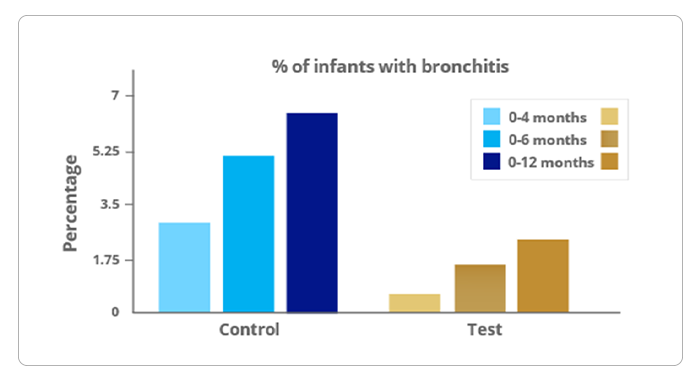
Results1
1.Bronchitis
- Infants had significantly fewer reports of bronchitis at 4, 6, and 12 months with test feed.
- Statistically significant difference was observed through 12 months in an infants’ subgroup born via c-section for bronchitis (test vs control; 3.1% vs. 34.4%).
Reduces the Risk of Bronchitis by 63%
2.Lower respiratory tract infections(LRTI)
- Statistically significant differences for LRTIs was observed in an infants’ subgroup born via cesarean section (test vs. control: at 6 months (6.3 % vs 28.1%) and at 12 months (12.5% vs 40.6%).
- Fewer parent-reported adverse event clusters with test feed through 12 months.
Lowers RTIs by 44%

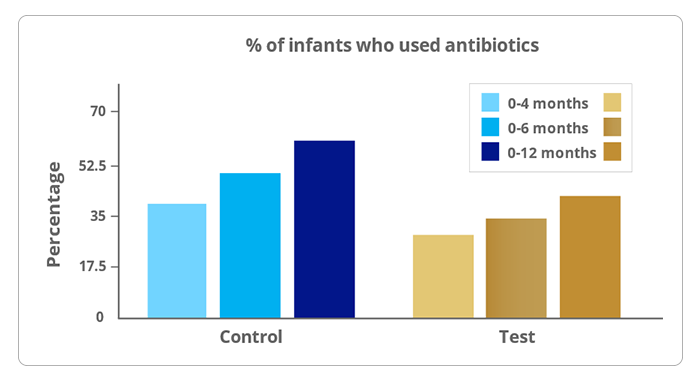
3.Antibiotic use
- Antibiotics usage was significantly lower through 6 and 12 months with test feed.
Reduces Antibiotic usage by 33%
4.Antipyretic use
- Reports of antipyretic usage was fewer through the first 4 months with test feed
Reduces Antipyretic usage by 21%

Conclusion
HMOs are a vital component of human milk, contributing to the overall well-being of infants. This randomized trial showed that infant feeds with HMO (2’-FL) are safe and well-tolerated in infants. HMOs help in improving immunity and fighting infections. Infants fed on feeds with HMO such as 2’-FL show lesser incidence of infections such as bronchitis and LRTIs. These infants also need fewer antipyretics and antibiotics to combat morbidities.
Reference:
- Puccio G, Alliet P, Cajozzo C, Janssens E, Corsello G, Sprenger N, Wernimont S, Egli D, Gosoniu L, Steenhout P. Effects of infant formula with human milk oligosaccharides on growth and morbidity: a randomized multicenter trial. Journal of pediatric gastroenterology and nutrition. 2017 Apr;64(4):624.
- Vandenplas Y, Berger B, Carnielli VP, Ksiazyk J, Lagström H, Sanchez Luna M, Migacheva N, Mosselmans JM, Picaud JC, Possner M, Singhal A. Human milk oligosaccharides: 2′-fucosyllactose (2′-FL) and lacto-N-neotetraose (LNnT) in infant formula. Nutrients. 2018 Aug 24;10(9):1161.
CTA: Dear doctor, do you agree that feed containing 2'-FLs will boost immunity and reduce infection burden in infants?
- Yes, definitely
- No, more evidences are required

