Effect of the specific infant formula mixture of oligosaccharides on local immunity and development of allergic and infectious disease in young children: randomized study
Study background
The type of diet plays a very important role in determining the intestinal flora of infants. The microflora of breastfed infants differs from formula-fed infants. The influence of breast milk on the development of infant’s immune system has been well known and is partly brought about by human milk oligosaccharides (HMOs) present in it. The gut microbiota of breastfed infants is predominantly dominated by and , and this microbial pattern produces beneficial effects on intestinal function and immune system.
Studies in preterm and term infants have shown that feed supplementation with scGOS:lcFOS confers immunity and produces an intestinal flora similar to that of breastfed infants. Several studies in preterm and term infants have indicated that use of scGOS: lcFOS helps:
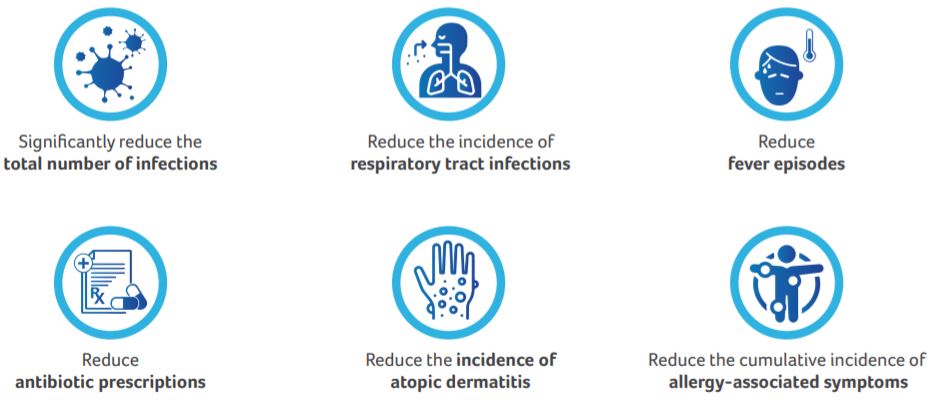
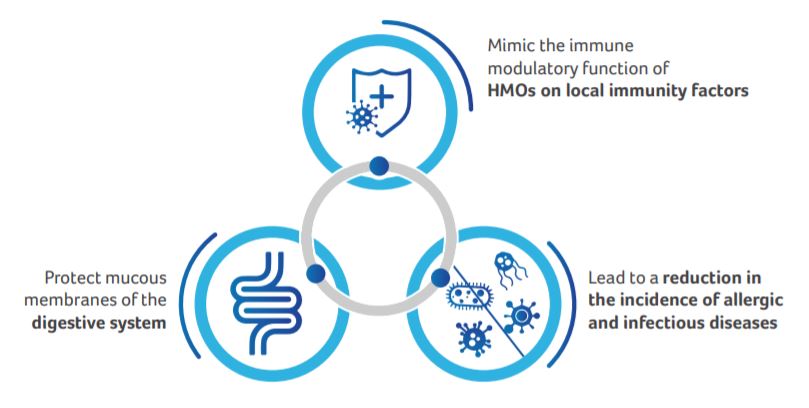
Based on the analysis of HMOs, a prebiotic mixture of 90% short-chain galacto-oligosaccharides and 10% long-chain fructo-oligosaccharides scGOS:lcFOS (9:1; 8 g/L) had been developed. The present study was conducted to test the hypothesis that the specific mixture of scGOS:lcFOS can:
Aim
The study was conducted to evaluate the effect of feeding with a standard infant formula enriched with the specific mixture of oligosaccharides scGOS:lcFOS; (9:1; 8 g/L) compared to a formula without oligosaccharides and breastfeeding during the first months of life on digestive system, local immunity and further development of allergic and infectious diseases.
Study design
The open-prospective, randomized intervention study allocated healthy term newborn infants into 3 intervention groups based on the type of feeding:
| Study group | Type of feeding |
|---|---|
| Group 1 | Infants on breast milk (n=80) |
| Group 2 | Formula supplemented with a specific mixture of oligosaccharides (scGOS:lcFOS; 9:1) (n=80) |
| Group 3 | Standard formula without prebiotics (n=80) |
Healthy newborn infants with birth weight >2500 g appropriate for gestational age were enrolled for the trial. The study period was inclusive of two follow-up phases. The first follow-up was at 2 months and second follow-up was at 18 months.
Parameters assessed:
- Growth (height & weight)
- Gut microbiota composition
- Cumulative incidences of allergies and infectious diseases
Result & Discussion
The study showed that scGOS:lcFOS supplementation influenced intestinal microbiota and could positively modulate infant's immune system development, and reduce some allergic and infectious morbidity in infants and toddlers aged up to 18 months.
Concentration of lysozyme in faeces
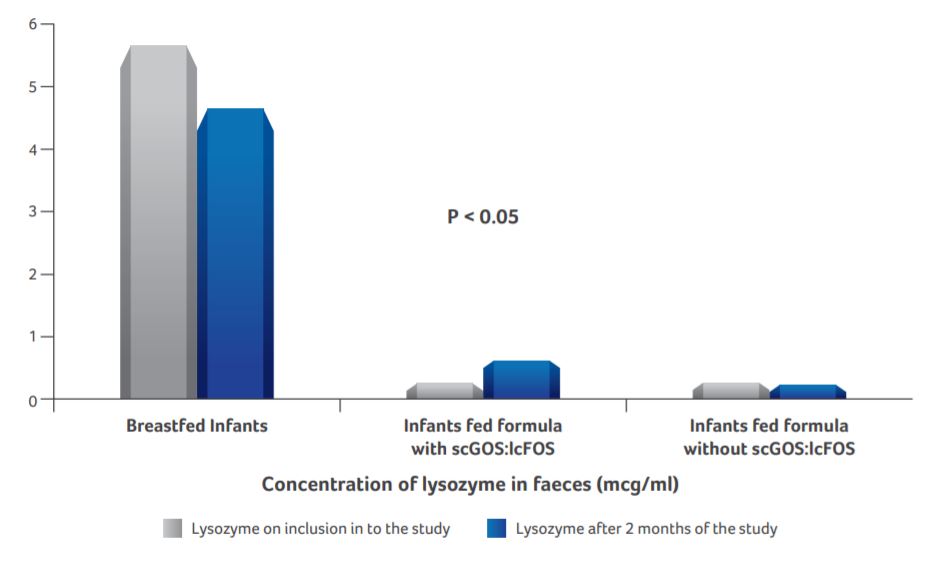
- Concentrations of faecal lysozyme were significantly lower at the inclusion into the study for infants fed with scGOS:lcFOS formula and standard formula as compared to the breastfed infants.
- Post 2 months of intervention, faecal lysozyme content was significantly (P < 0.05) higher in infants supplemented with scGOS:lcFOS formula than infants fed standard formula.
Saliva concentration of SIgA
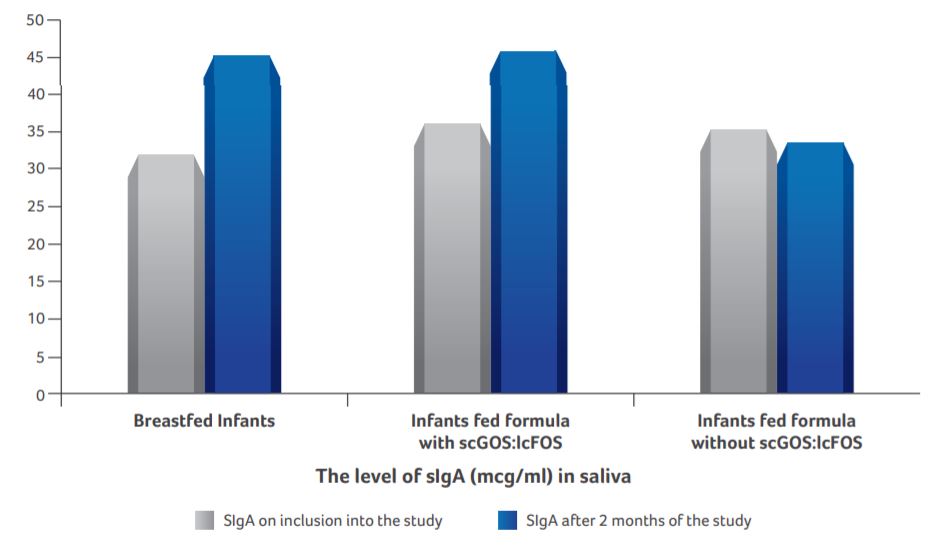
- Saliva concentration of SIgA in infants supplemented with scGOS:lcFOS was similar to that of the breastfed infants (P < 0.05).
- No obvious changes were observed in infants fed standard formula.
Saliva concentrations of α-1-3 defensins
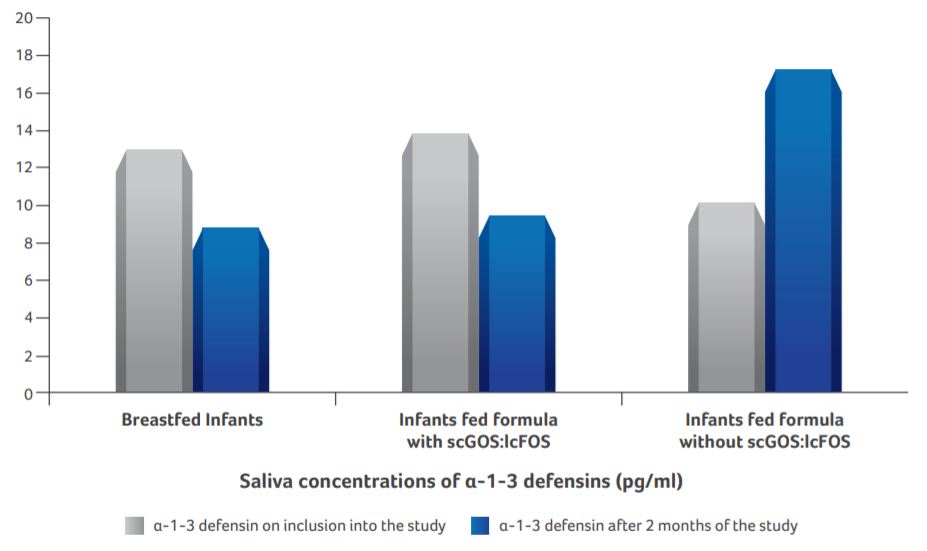
- Breastfed infants demonstrated the lowest level of α-1-3 defensin concentration.
- Defensins concentration in infants supplemented with scGOS:lcFOS formula was similar to that of breastfed infants (P < 0.01).
- The increased level of α-1-3 defensins produced by neutrophils in infants supplemented with standard formula (without scGOS:lcFOS) may be a result of protective distress of immune reactions that indicate formation of pathological bacterial gut colonization.
Parents of 195 infants agreed to participate in stage 2 of the trial (follow-up at 18 months) and the following observations were made:
Incidence of infections
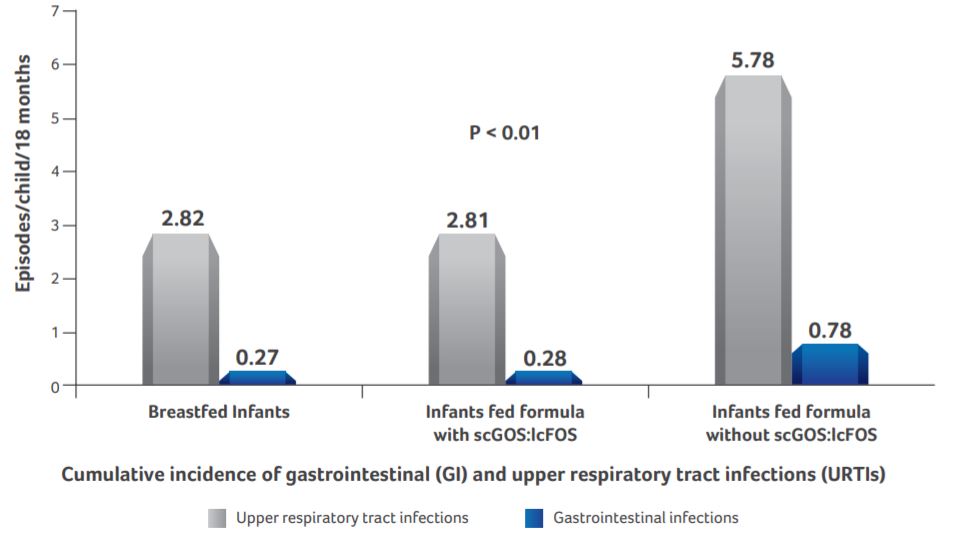
- Breastfed infants and infants supplemented with scGOS:lcFOS formula had similar morbidity (0.27±0.07 vs. 0.28±0.05 episodes/child/18 months of gastrointestinal infections and 2.82±0.96 vs. 2.81±0.51 episodes/child/18 months of URTI respectively.)
- On the other hand, the incidence of gastrointestinal and upper respiratory tract infections in infants supplemented with scGOS:lcFOS formula was significantly lower than in infants fed standard formula (0.28±0.05 vs. 0.78±0.12 episodes/child/18 months and 2.81±0.51 vs. 5.78±0.97 episodes/child/18 months accordingly).
Allergic reactions to food products
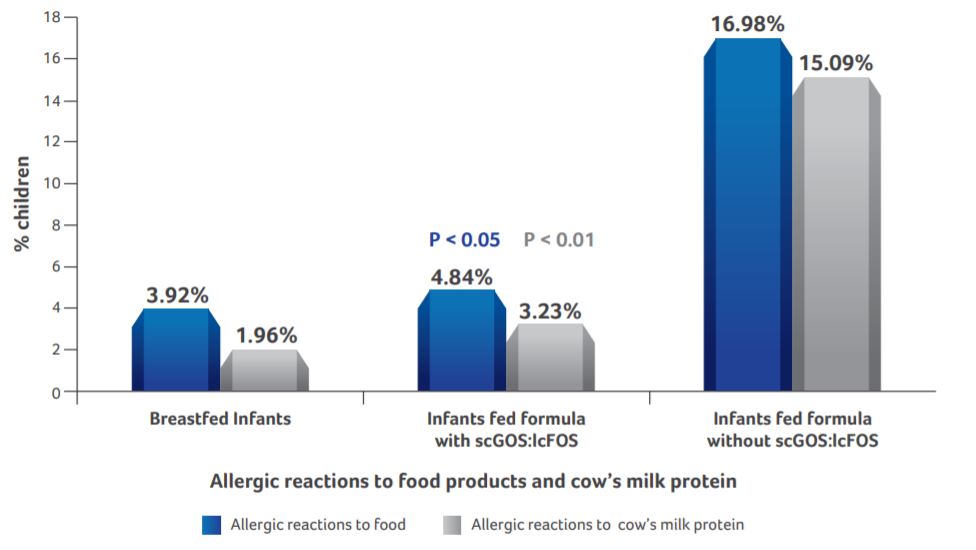
- Breastfed infants and infants supplemented with scGOS:lcFOS formula had significantly lesser allergic reactions to food products as compared to infants fed standard formula (P < 0.05).
- Allergic reactions to cow’s milk protein was significantly lower in breastfed infants and infants supplemented with scGOS:lcFOS formula as compared to infants fed standard formula (P < 0.01).
Incidence of atopic dermatitis
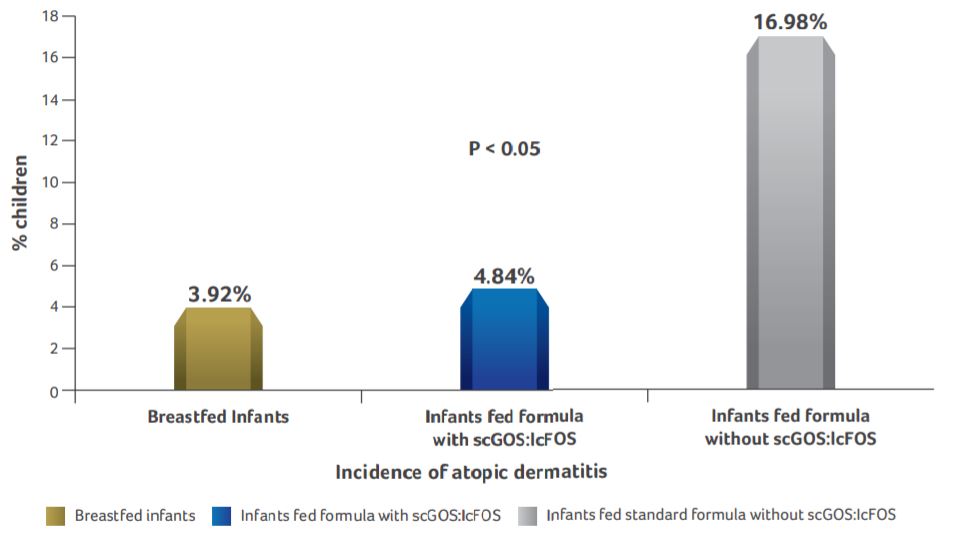
- Incidence of atopic dermatitis was significantly higher in infants fed standard formula as compared to breastfed infants and infants supplemented with scGOS:lcFOS formula.
Conclusion
- Early dietary intervention with oligosaccharide prebiotics has been found to have a protective effect against both allergic manifestations and infections.
- The trial showed that supplementation of infant formula with a specified mixture of scGOS:lcFOS in the ratio of 9:1 positively influenced intestinal microbiota, modulated infant’s immune system development and reduced some allergic and infectious morbidity in infants up to 18 months.
- Infants supplemented with scGOS:lcFOS formula demonstrated a beneficial antibody profile i.e. had fewer episodes of physician-diagnosed overall and upper respiratory tract infections, fever episodes, and fewer antibiotic prescriptions.
Key Findings
- Supplementation of an infant formula with a prebiotic mixture of oligosaccharides (scGOS:lcFOS) in the ratio of 9:1 has a similar positive impact as breast milk on factors of local protection of mucous membranes such as lysozyme, α-defensins 1-3, SIgA, intestinal microbiota in formula-fed infants.
- Effect of prebiotic oligosaccharides helps reduce the risk of allergic and infectious diseases in children aged up to 18 months of life.
References:
- Ivakhnenko OS., and Nyankovskyy SL. Effect of the specific infant formula mixture of oligosaccharides on local immunity and development of allergic and infectious disease in young children: A randomized study. Pediatia Polska (2013) 398 – 404.

