
Effect of Human Milk Oligosaccharides on Infant Gut
Highlights
- Human milk oligosaccharides (HMOs) are the third most abundant component present in human milk.
- HMOs, such as 2’-FL, exhibit several health benefits for infants. It can modulate gut microbiota and immune responses in the intestinal mucosa.
- 2′-FL increases Bifidobacteria colonization and reduces the risk of developing IgE-associated allergies.
This article discusses the current insight related to the functional biology of HMOs and their associated effect on infants’ gut health.
The gut microbiota is crucial for metabolism and immunity; its establishment after birth determines overall health and immunity later in life. The sources of microbes for the neonatal gut are the mother’s skin, breastmilk, along with the extrauterine environment. Factors like gestational age at birth, mode of delivery (vaginal or cesarean), mode and type of feeding, antibiotic use, maternal diet, and health have the ability to affect an infant’s gut microflora. For about six months after birth, the mother's milk is the primary food source for the baby.1
Human milk oligosaccharides (HMOs) are the third most abundant component in human milk, following lactose and lipids.2 2′-fucosyllactose (2′-FL) is the most abundant HMO, accounting for almost 30% of HMOs in human milk.2 HMOs present various health benefits to infants by impacting their gut, as discussed below (Figure 1).2
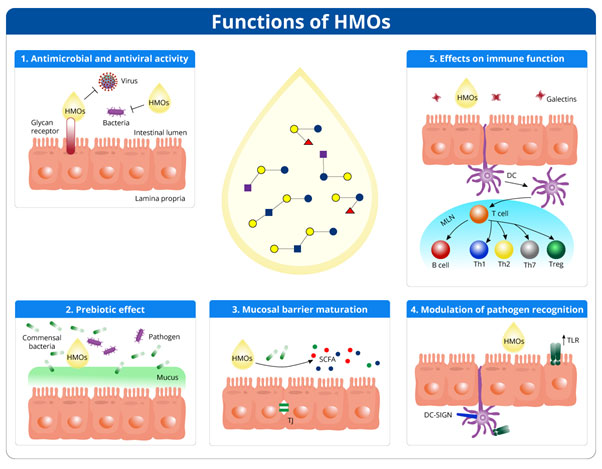
Figure 1: Figure 1. Effect of HMOs on infant’s gut (Adapted from Ayechu-Muruzabal V et al., 2018)3
Anti-infective effect
HMOs display anti-microbial and anti-viral effects and prevent infections in breastfed infants. The mechanisms for anti-infective effects are:3
- Binding to viruses, bacteria, toxins, or eukaryotes
- Mimicking pathogen receptors and blocking their entry into host cells
- Blocking viral replication inside host cells
HMOs have been reported to have anti-infective properties against pathogens such as:3
- Norovirus
- Rotavirus
- Escherichia coli
- Group B Streptococci
- Campylobacter jejuni
Furthermore, α1-2-fucosylated HMO can reduce the incidence of C. jejuni-associated diarrhea.4
Prebiotic effect
HMOs are naturally occurring prebiotics present in breast milk. HMOs act as an energy source for gut bacteria and influence intestinal microbiota.2 They exert selective prebiotic effects on an infant’s gut by promoting the growth of beneficial bacteria and inhibiting harmful bacteria. Thus, HMOs help in reducing the chances of infection in infants.3 Table 1 enlists the microbes modulated by HMOs.3 In 3-month-old infants, supplementation with 2′-FL and other HMO is associated with increased bifidobacterial and decreased pathogenic bacterial colonization.2
Table 1. Microbiota modulation by HMOs3
|
Microbes promoted by HMOs |
Microbes inhibited by HMOs |
|
Bifidobacteria spp. |
E. coli |
|
Bacteroides spp. |
Clostridia |
|
Eubacteria |
|
|
Enterococci |
Mucosal barrier maturation
HMOs and their metabolites, short-chain fatty acids (SCFA), stimulate gut maturation and epithelial barrier integrity in infants' gut. HMOs support mucosal barrier maturation by interacting with dendritic cells or glycans on epithelial cells in the intestinal mucosa. Additionally, HMOs induce differentiation of epithelial cells.3
Modulation of pathogen recognition
HMOs increase the expression of pathogen recognition receptors such as toll-like receptors (TLRs) and stimulate TLR signaling pathways. In addition, HMOs target C-type lectins, which are also crucial in pathogen recognition. Eventually, immune homeostasis is also modulated by HMOs.3
Effect on immune system development
Gut-associated lymphoid tissue (GALT) is a secondary lymphoid organ that processes the antigens interacting with the intestinal mucosa and disseminates the immune response.5 HMOs alter the expression of glycans and cell responses of intestinal cells. Moreover, they regulate inflammatory cytokine production by lymphocytes. HMOs directly inhibit inflammation and stimulate the innate immune system. Feeds with 2′-FL reduce the risk of immunoglobulin E-associated allergies.2
Effect on gut functioning
HMOs support healthy gut development in infants. Furthermore, feeds with 2′-FL and other HMOs help to develop a bacterial profile of stool similar to exclusively breastfed infants.2 Bifidobacteria present in gut regulate carbohydrate metabolism, especially HMOs.6 SCFAs produced by bifidobacteria improve absorption of calcium and magnesium and lowers gut pH.6
Conclusion
HMOs are one of the most significant components of human milk. They have anti-pathogenic effects, prevent infections in infants, and selectively promote the growth of beneficial microbes. Additionally, HMOs can facilitate mucosal barrier function and immune system development.2
References
- Bharadia L, Agrawal N, Joshi N. Development and Functions of the Infant Gut Microflora: Western vs. Indian Infants. Int J Pediatr. 2020 May 7;2020:7586264.
- Hegar B, Wibowo Y, Basrowi RW, Ranuh RG, Sudarmo SM, Munasir Z, Atthiyah AF, Widodo AD, Supriatmo, Kadim M, Suryawan A, Diana NR, Manoppo C, Vandenplas Y. The Role of Two Human Milk Oligosaccharides, 2'-Fucosyllactose and Lacto-N-Neotetraose, in Infant Nutrition. Pediatr Gastroenterol Hepatol Nutr. 2019 Jul;22(4):330-340.
- Ayechu-Muruzabal V, van Stigt AH, Mank M, Willemsen LEM, Stahl B, Garssen J, Van't Land B. Diversity of Human Milk Oligosaccharides and Effects on Early Life Immune Development. Front Pediatr. 2018 Sep 10;6:239.
- Vandenplas Y, Berger B, Carnielli VP, Ksiazyk J, Lagström H, Sanchez Luna M, Migacheva N, Mosselmans JM, Picaud JC, Possner M, Singhal A, Wabitsch M. Human Milk Oligosaccharides: 2'-Fucosyllactose (2'-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients. 2018 Aug 24;10(9):1161.
- Plaza-Díaz J, Fontana L, Gil A. Human Milk Oligosaccharides and Immune System Development. Nutrients. 2018 Aug 8;10(8):1038.
- O'Callaghan A, van Sinderen D. Bifidobacteria and Their Role as Members of the Human Gut Microbiota. Front Microbiol. 2016 Jun 15;7:925.
1664508340371





